Smart Drug Delivery
Research Focus
Wheat germ agglutinin (WGA) is a dietary lectin that adheres to the mucus as well as the membrane of absorptive cells and it is taken up into the cells. For smart drug delivery the question arises whether WGA can carry drug delivery systems as a backpack like a “Sherpa” to or into absorptive cells.
By mimicking the adhesion of uropathogenic E. coli on the urothelium, smart nanopharmaceuticals consisting of a drug loaded core and a WGA-corona, a new strategy for treatment of urinary tract infections and cancer by instillative administration is currently investigated.
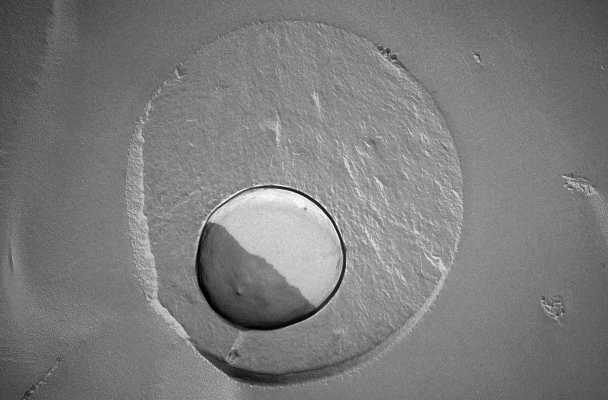
Scanning electron microscopic image of a human serum albumin nanocapsule with an oily core.
Methods
Techniques applied comprise preparation and characterization of nanoparticles, cultivation of cells, interaction of cells with micro- and nanoformulations, HPLC, fluorescence microscopic and co-localisation techniques.
Selected publications
Apfelthaler, C., Anzengruber, M., Gabor, F., Wirth, M., Poly – (L) – glutamic acid drug delivery system for the intravesical therapy of bladder cancer using WGA as targeting moiety. European J. Pharm. Biopharm., 115, 131-139 (2017).
Apfelthaler, C., Gassenbauer, P., Weisse, S., Gabor, F., Wirth, M., A lectin mediated delivery system for the intravesical treatment of bladder diseases using poly-(L)-glutamic acid as polymeric backbone. European Journal of Pharmaceutical Sciences, 111, 376-382 (2018).
Apfelthaler, C., Skoll, K., Ciola, R., Gabor, F., Wirth, M., A doxorubicin loaded colloidal delivery system for the intravesical therapy of non-muscle invasive bladder cancer using wheat germ agglutinin as targeter. European Journal of Pharmaceutical Sciences, 130, 177-184 (2018).
Brauner. B., Schuster, C., Wirth, M., Gabor, F., Trimethoprim-loaded microspheres prepared from low-molecular-weight PLGA as a potential drug delivery system for the treatment of urinary tract infections. ACS Omega 5, 15, 9013-9022 (2020); (https://doi.org/10.1021/acsomega.0c00981).
Brauner. B., Schwarz, P., Wirth, M., Gabor, F., Micro vs. nano: PLGA particles loaded with trimethoprim for instillative treatment of urinary tract infections. Int J Pharm 579, 119-158 (2020); (https://doi.org/10.1016/j.ijpharm.2020.119158).
Brauner. B., Semmler, J., Rauch, D., Nokaj, M., Haiss, P., Schwarz, P., Wirth, M., Gabor, F., Trimethoprim-loaded PLGA nanoparticles grafted with WGA for intravesical therapy of urinary tract infections – studies on adhesion to SV-HUC cells under varying time, pH and drug loading conditions. ACS Omega 2020; 5, 28,17377-17384 (https://doi.org/10.1021/acsomega.0c01745).
Skoll, K., Ritschka, M., Fuchs, S., Wirth, M., Gabor, F. Characterization of sonochemically prepared human serum albumin nanocapsules using different plant oils as core component for targeted drug delivery. Ultrasonics Sonochemistry 2021 Aug; 76:105617 (https://doi.org/10.1016/j.ultsonch.2021.105617).
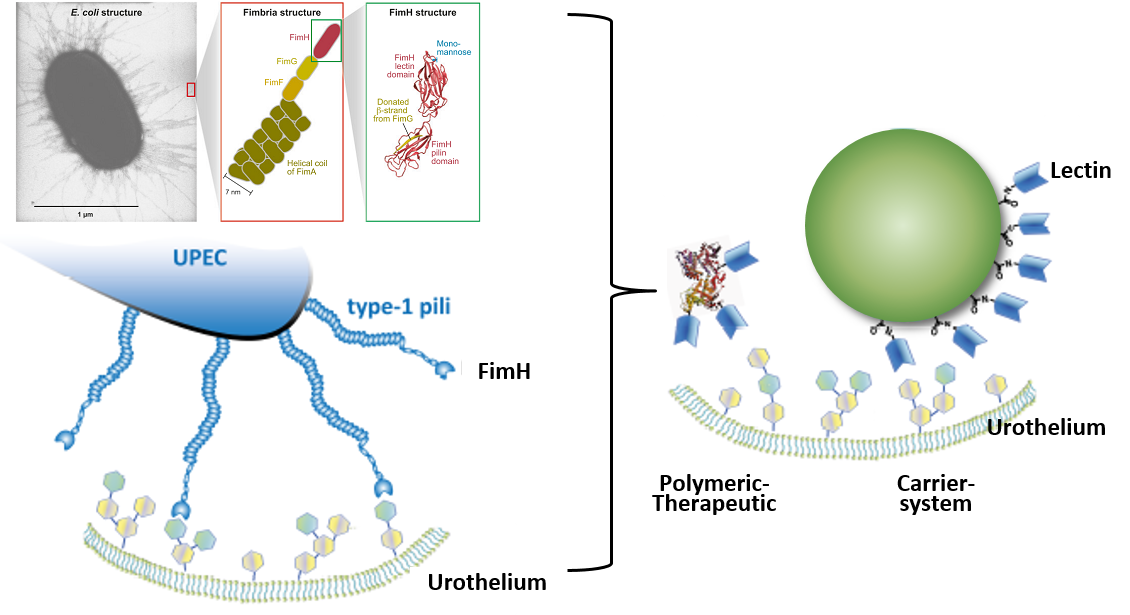
Biomimesis of the interaction of uropathogenic Escherichia coli and WGA-grafted formulations with the urothelium.
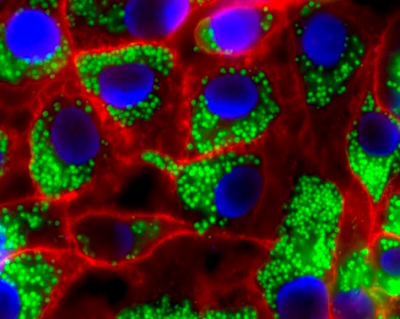
Interaction of WGA-grafted nanoparticles (green) with artificial cancerous epithelium (nuclei blue, cell membrane red).
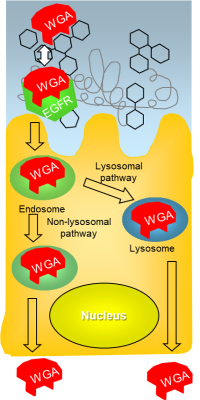
Interaction of Wheat germ agglutinin with absorptive cells.
