Nanomedicine and pharmaceutical biophysics
Group aims
Our research group focuses on different aspects of the formulation and characterization of nanomedicines for the delivery to the respiratory system, lung toxicology of nanoplastics and biophysical studies of colloids for membrane-related studies and drug delivery purposes.
Research projects
- Imptox: an innovative analytical platform to investigate the effect and toxicity of micro and nano plastics (Prof. Dr. Lea Ann Dailey, Mr. Mag. Lukas Wimmer, Ms. My Vanessa Nguyen Hoang, BSc)
Plastic is exceptionally durable and can persist in the environment, where it undergoes slow degradation. When plastics degrade, they form microplastic and sub-micron-sized nanoplastic particles (MNPs). The burden of MNPs in the environment is currently unknown, due to a lack of analytical tools to measure MNPs in complex environmental matrices. The Imptox project, funded by the EU’s Horizon 2020 programme, will investigate the impact of MNPs on human health and allergy.
Our role in the project is to optimise manufacturing methods to generate reference micro- and nanoplastics, which will then be sent to our project partners. In addition, micro- and nanoplastics present in sea spray aerosol will be collected and investigated with cutting-edge analytical methods, such as AFM-IR, to understand respiratory exposure.
http://www.imptox.eu/
- Novel anti-infectives for lung diseases (Prof. Dr. Lea Ann Dailey, Ms. Dr. Gabriela Hädrich, Ms. Mag. Jacqueline Schwarzinger and Ms. Feng Li, MSc BSc; in collaboration with the research group of Prof. Dr. Judith M. Rollinger and Dr. Ulrike Grienke, Division of Pharmacognosy, University of Vienna)
To successfully treat lung diseases with anti-infectives given intrapulmonary the compound must be presented in a way that it should not diffuse quickly to the blood stream and avoid the toxicity of local accumulation. To find this ideal balance, in vitro and in vivo studies are needed to better understand the compound’s behaviour and propose a target product profile. For that, our group is equipped with a Next Generation Impactor (NGI; Copley) to study lung deposition in vitro, cell culture for permeation studies and a PreciseInhale® (Inhalation Sciences) in vivo studies.
- ANTI-TB – Antibiotics nanocarrier for therapeutic inhalation against tuberculosis (Prof. Dr. Lea Ann Dailey, Ms. Dr. Gabriela Hädrich, Ms. Feng Li, MSc BSc)
According to the WHO, globally, an estimated 10.0 million people fell ill with TB in 2019, a number that has been declining very slowly in recent years. The COVID-19 pandemic has reversed years of progress in providing essential TB services and reducing TB disease burden. The impacts include reductions between 2019 and 2020 in the number of people provided with treatment for drug-resistant TB and TB preventive treatment, and a fall in global spending on TB diagnostic, treatment and prevention services. The TB standard-of-care antibiotic treatment is required to be given for at least 6 months but is often accompanied by significant adverse effects, which can lead to premature termination of the therapy. Thereby, the occurrence of antibiotic-resistant strains is promoted further hampering successful therapy.
The ANTI-TB research consortium received 2.8 million Euros of funding from the German Federal Ministry of Education and Research (BMBF). The aim of the project was use nanocarriers to improve therapies of antibiotic resistant tuberculosis. The project partners include the Borstel Research Centre, the Leibniz Institute for Medicine and Biosciences (FZB), the Karlsruhe Institute of Technology (KIT), the Fraunhofer Institute for Toxicology and Experimental Medicine Hanover (ITEM), the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), the Martin Luther University Halle-Wittenberg (MLU-HW) as well as the biopharmaceutical company Rodos Biotarget GmbH (RBT).
Prof. Dailey, Dr. Hädrich and Ms. Li conducted pharmacokinetics studies aiming to better understand if the antibitiocs when entrapped into nanocarriers can have an advantage when administered via inhalation. Now at UNIVIE the researchers are performing the final stage of this project, analyzing the data and writing the scientific reports.
http://antitb.fz-borstel.de/
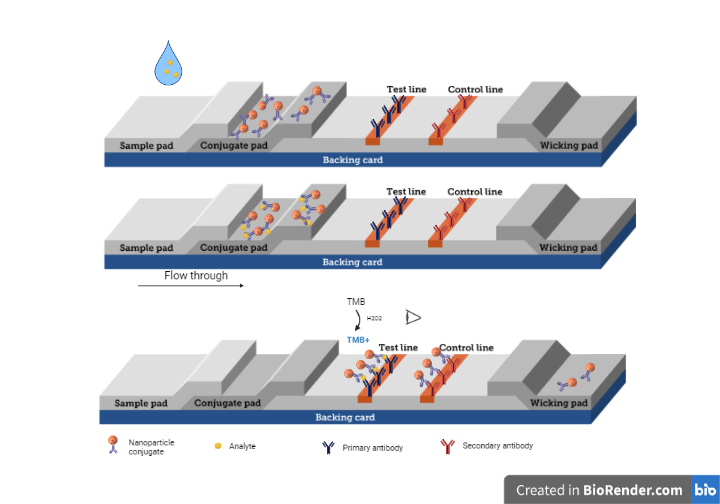
- Investigation of high entropy nanoparticles as nanozymes to improve sensitivity of lateral flow immunoassays (Prof. Dr. Lea Ann Dailey, Mr. Thuong Phan Xuan, Msc)
Lateral Flow Immunoassays (LFIAs) have become more popular for low-cost, easy-to-use, rapid testing in point-of-care (POC) applications. They are promising tools for rapid screening of many viral and bacterial infections, thus offer a good control of pandemic at early stage. However, the limit of detection of LFIAs is still an obstacle, compare to other diagnostic tools, such as PCR. Since the peroxidase-mimicking activity of Fe3O4 nanoparticles was reported in 2007, various number of enzyme-like nanomaterials (called nanozymes) have been studied as new signal labels in LFIAs. High entropy nanomaterials (HENs) have drawn attention due to their unique characteristics. They have been investigated for various applications in energy, environment, material sciences. However, their utility in biomedical research is of question. The aim of our study is to determine the enzyme-mimicking properties of HENs as well as their potential as signal labels which can offer an improved sensitivity in LFIAs.
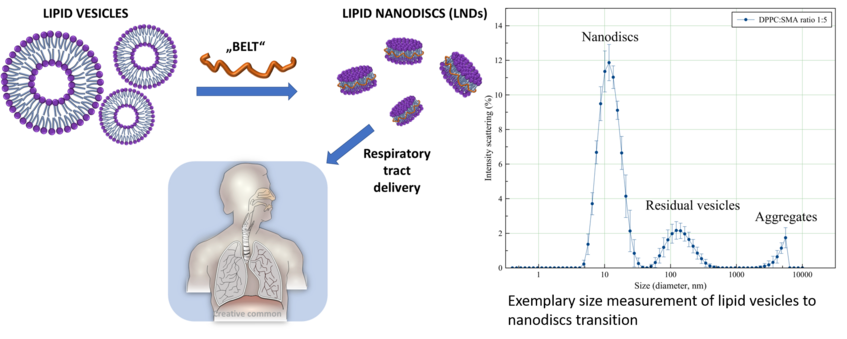
- Nanodiscs research (Mr. Dr. Gianluca Bello)
Lipid nanodiscs (LNDs) have been recently developed as efficient membrane protein purification tool and suitable matrix for membrane protein research. In this project, the functionality of nanodiscs is expanded by using biocompatible polymeric membranes and uncommon amphiphiles. The biophysical characterization of such nanodiscs aims to define possible applications such as the respiratory tract drug delivery of functional membrane proteins or poorly soluble molecules. X-ray, spectroscopy, imaging and interface studies are the techniques of choice for this purpose.
- Lipid biophysics (Mr. Dr. Richard Harvey)
Characterisation of mixing properties, phase morphology and bio-interfacial interactions of natural lipids implicated in intrinsic antibiotic resistance and synthetic lipids commonly used in drug delivery. The development of membrane mimetic models for studying drug adsorption and uptake.
- Membrane activity of SARS-CoV E-protein (Mr. Dr. Richard Harvey and Mr. Dr. Gianluca Bello)
This project focuses on understanding the role of the E-protein from SARS-CoV in the viral replication cycle. Specifically, E-protein hijacks the ERGIC membrane system of a cell to build up its envelope. By applying X-ray techniques, we aim to elucidate the structural changes induced by E-protein on the ERGIC membrane system for possible therapeutic target applications.
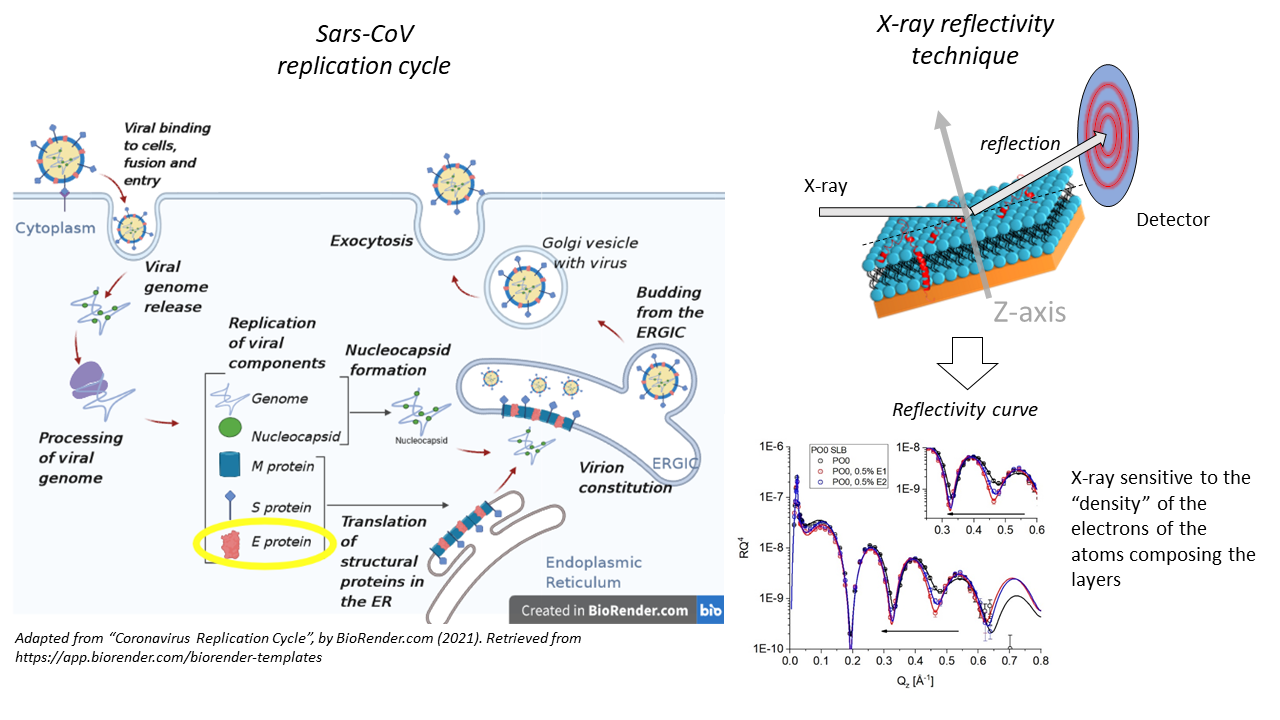
Methods
UV spectrometry, Isothermal titration calorimetry (ITC), dynamic light scattering (DLS), Langmuir monolayers (LM), cell and bacterial culture (BSL2), small angle and reflectivity X-ray and neutron experimental planning and actuation in specialized facilities (i.e. DESY, ILL, Rutherford Appleton laboratories), lipid nanoemulsion (LNE) formulation, atomic force microscopy (AFM), NMR, HPLC, GC, quartz crystal microbalance with dissipation (QCM-D).
Instruments
BSL2 microbiology laboratory and cell culture, Kibron Langmuir trough, Malver zetasizer DLS, TA instruments ITC, Epoc 2 (Biotek) spectrophotometer, Next generation impactor & Twin stage impinger (Copley), PreciseInhale (Inhalation Sciences).
List of publications
Li, F.; Harvey, R. D.; Modicano, P.; Hamdi, F.; Kyrilis, F.; Müller, S.; Gruhle, K.; Kastritis, P.; Drescher, S.; Dailey, L. A. Investigating Bolalipids as Solubilizing Agents for Poorly Soluble Drugs: Effects of Alkyl Chain Length on Solubilization and Cytotoxicity. Colloids Surfaces B Biointerfaces 2022, 212, 112369. https://doi.org/10.1016/j.colsurfb.2022.112369.
Neumann, P. R.; Erdmann, F.; Holthof, J.; Hädrich, G.; Green, M.; Rao, J.; Dailey, L. A. Different PEG‐PLGA Matrices Influence In Vivo Optical/Photoacoustic Imaging Performance and Biodistribution of NIR‐Emitting π ‐Conjugated Polymer Contrast Agents. Adv. Healthc. Mater. 2021, 10 (4), 2001089. https://doi.org/10.1002/adhm.202001089.
Schüller, M.; Meister, A.; Green, M.; Dailey, L. A. Investigating Conjugated Polymer Nanoparticle Formulations for Lateral Flow Immunoassays. RSC Adv. 2021, 11 (47), 29816–29825. https://doi.org/10.1039/d1ra05212h.
Hädrich, G.; Dora, C. L.; Vaz, G. R.; Boschero, R.; Appel, A. S.; Ramos, C.; Halicki, P. C. B.; Bidone, J.; Teixeira, H. F.; Muccillo-Baisch, A. L.; Dal-Bó, A.; da Silva Pinto, L.; Dailey, L.-A.; Da Silva, P. E. A.; Soares, D. R. Development of Lipid Nanocarriers for Tuberculosis Treatment: Evaluation of Suitable Excipients and Nanocarriers. Curr. Drug Deliv. 2021, 18. https://doi.org/10.2174/1567201818666210212092112.
Chan, K. L. A.; Lekkas, I.; Frogley, M. D.; Cinque, G.; Altharawi, A.; Bello, G.; Dailey, L. A. Synchrotron Photothermal Infrared Nanospectroscopy of Drug-Induced Phospholipidosis in Macrophages. Anal. Chem. 2020, 92 (12), 8097–8107. https://doi.org/10.1021/acs.analchem.9b05759.
Vandera, K. K. A.; Picconi, P.; Valero, M.; González-Gaitano, G.; Woods, A.; Zain, N. M. M.; Bruce, K. D.; Clifton, L. A.; Skoda, M. W. A.; Rahman, K. M.; Harvey, R. D.; Dreiss, C. A. Antibiotic-in-Cyclodextrin-in-Liposomes: Formulation Development and Interactions with Model Bacterial Membranes. Mol. Pharm. 2020, 17 (7), 2354–2369. https://doi.org/10.1021/acs.molpharmaceut.0c00096.
Hädrich, G.; Boschero, R. A.; Appel, A. S.; Falkembach, M.; Monteiro, M.; da Silva, P. E. A.; Dailey, L. A.; Dora, C. L. Tuberculosis Treatment Facilitated by Lipid Nanocarriers: Can Inhalation Improve the Regimen? Assay Drug Dev. Technol. 2020, 18 (7), 298–307. https://doi.org/10.1089/adt.2020.998.
Patel, A.; Redinger, N.; Richter, A.; Woods, A.; Neumann, P. R.; Keegan, G.; Childerhouse, N.; Imming, P.; Schaible, U. E.; Forbes, B.; Dailey, L. A. In Vitro and in Vivo Antitubercular Activity of Benzothiazinone-Loaded Human Serum Albumin Nanocarriers Designed for Inhalation. J. Control. Release 2020, 328, 339–349. https://doi.org/10.1016/j.jconrel.2020.08.022.
Modicano, P.; Neumann, P. R.; Schüller, M.; Holthof, J.; Kyrilis, F. L.; Hamdi, F.; Kastritis, P. L.; Mäder, K.; Ann Dailey, L. Enhanced Optical Imaging Properties of Lipid Nanocapsules as Vehicles for Fluorescent Conjugated Polymers. Eur. J. Pharm. Biopharm. 2020, 154 (July), 297–308. https://doi.org/10.1016/j.ejpb.2020.07.017.
Bello, G.; Cavallini, F.; Dailey, L. A.; Ehmoser, E. K. Supported Polymer/Lipid Hybrid Bilayers Formation Resembles a Lipid-like Dynamic by Reducing the Molecular Weight of the Polymer. Biochim. Biophys. Acta - Biomembr. 2020, 1863 (1), 183472. https://doi.org/10.1016/j.bbamem.2020.183472.
Marbach, H.; Vizcay-Barrena, G.; Memarzadeh, K.; Otter, J. A.; Pathak, S.; Allaker, R. P.; Harvey, R. D.; Edgeworth, J. D. Tolerance of MRSA ST239-TW to Chlorhexidine-Based Decolonization: Evidence for Keratinocyte Invasion as a Mechanism of Biocide Evasion. J. Infect. 2019, 78 (2), 119–126. https://doi.org/10.1016/J.JINF.2018.10.007.
Pabois, O.; Lorenz, C. D.; Harvey, R. D.; Grillo, I.; Grundy, M. M. L.; Wilde, P. J.; Gerelli, Y.; Dreiss, C. A. Molecular Insights into the Behaviour of Bile Salts at Interfaces: A Key to Their Role in Lipid Digestion. J. Colloid Interface Sci. 2019, 556, 266–277. https://doi.org/10.1016/J.JCIS.2019.08.010.
Schmid, M.; Wölk, C.; Giselbrecht, J.; Chan, K. L. A.; Harvey, R. D. A Combined FTIR and DSC Study on the Bilayer-Stabilising Effect of Electrostatic Interactions in Ion Paired Lipids. Colloids Surfaces B Biointerfaces 2018, 169, 298–304. https://doi.org/10.1016/J.COLSURFB.2018.05.031.
Hubbard, A. T. M.; Barker, R.; Rehal, R.; Vandera, K. K. A.; Harvey, R. D.; Coates, A. R. M. Mechanism of Action of a Membrane-Active Quinoline-Based Antimicrobial on Natural and Model Bacterial Membranes. Biochemistry 2017, 56 (8), 1163–1174. https://doi.org/10.1021/acs.biochem.6b01135.
Bixner, O.; Bello, G.; Virk, M.; Kurzhals, S.; Scheberl, A.; Gal, N.; Matysik, A.; Kraut, R.; Reimhult, E. Magneto-Thermal Release from Nanoscale Unilamellar Hybrid Vesicles. ChemNanoMat 2016, 2 (12), 111–1120. https://doi.org/10.1002/cnma.201600278.
Bello, G.; Bodin, A.; Jayne Lawrence, M.; Barlow, D.; James Mason, A.; Barker, R. D.; Harvey, R. D. The Influence of Rough Lipopolysaccharide Structure on Molecular Interactions with Mammalian Antimicrobial Peptides. Biochim. Biophys. Acta - Biomembr. 2015, 1858 (2), 197–209. https://doi.org/10.1016/j.bbamem.2015.11.007.


