Intracellular drug delivery
Our research focusses on the synthesis, characterization and application of nanopharmaceuticals for the delivery of drugs and biomolecules across cellular barriers.
Pharmacokinetic properties are inherent characteristics of drug molecules that cannot be changed without derivatization. The utilization of nanopharmaceuticals with tunable physicochemical properties is a rational approach to change the pharmacokinetics of drug molecules without affecting their biological activity. In this regard, controlled biodistribution does not only include differential extracellular tissue or organ accumulation, but also facilitated translocation across cellular barriers.
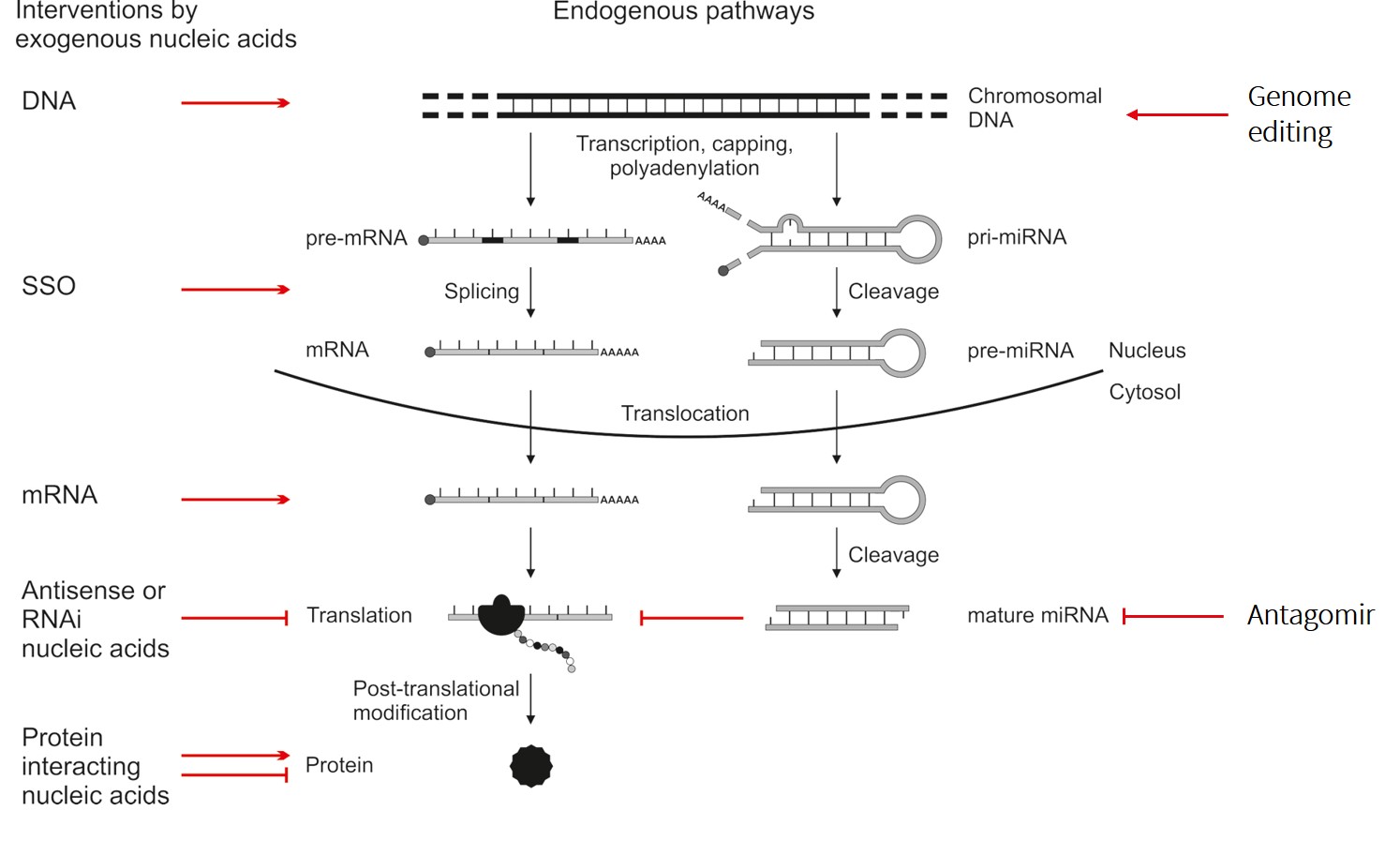
Flow of genetic information from chromosomal DNA to proteins and associated possibilities for therapeutic intervention by exogenous biomolecules. Reprinted with permission from Lächelt & Wagner, Chem. Rev. 2015, 115, 19, 11043–11078. Copyright 2015 American Chemical Society.
Different types of nucleic acids and genome editing components are versatile tools for therapeutic interventions based on their precise manipulation of the intracellular flow of genetic information. Unfortunately, the high therapeutic potential of biomacromolecules is generally opposed by a rather low ‘drug-likeness’ and unfavorable pharmacokinetic properties, in particular poor distribution to intracellular target sites.
To address these challenges, we develop nanopharmaceuticals for impermeable drugs and biomolecules based on peptides and metal-organic nanomaterials.

Methods
Peptide synthesis, metal-organic nanoparticle synthesis, physicochemical nanoparticle characterization, cellular uptake and bioactivity studies, molecular biology.
Selected Publications
Lessl A.-L., Pöhmerer J., Lin Y., Wilk U., Höhn M., Hörterer E., Wagner E., Lächelt U.*, mCherry on Top: A Positive Read-Out Cellular Platform for Screening DMD Exon Skipping Xenopeptide–PMO Conjugates, Bioconjugate Chem., 2023.
Albuquerque L.J.C., de Oliveira F.A., Christoffolete M.A., Nascimento-Sales M., Berger S., Wagner E., Lächelt U., Giacomelli F.C.*, Nucleic acid delivery to retinal cells using lipopeptides as a potential tool towards ocular gene therapies, J. Colloid Interface Sci., 2024, 665, 346-356.
Hall A., Bartek J., Wagner E., Lächelt U.*, Moghimi S.M.*, High-resolution bioenergetics correlates the length of continuous protonatable diaminoethane motif of four-armed oligo(ethanamino)amide transfectants to cytotoxicity, J. Control. Release, 2023, 361, 115-129.
Lin Y., Luo X., Burghardt T., Dorrer S., Höhn M., Wagner E.*, Lächelt U.*, Chemical Evolution of Amphiphilic Xenopeptides for Potentiated Cas9 Ribonucleoprotein Delivery, J. Am. Chem. Soc., 2023, 145, 28, 15171–15179
Lin Y., Wilk U., Pöhmerer J., Hörterer E., Höhn M., Luo X., Mai H., Wagner E.*, Lächelt U.*, Folate Receptor-Mediated Delivery of Cas9 RNP for Enhanced Immune Checkpoint Disruption in Cancer Cells, Small, 2022, 2205318.
Dizaji N.M., Lin Y., Bein T., Wagner E., Wuttke S., Lächelt U.*, Engelke H.*, Biomimetic Mineralization of Iron-Fumarate Nanoparticles for Protective Encapsulation and Intracellular Delivery of Proteins, Chem. Mater., 2022, 34(19), 8684-8693.
Lin Y., Wagner E.*, Lächelt U.*, Non-viral delivery of the CRISPR/Cas system: DNA versus RNA versus RNP, Biomater. Sci., 2022, 10, 1166-1192.
Ettlinger R.*, Lächelt U., Gref R., Horcajada P., Lammers T., Serre C., Couvreur P., Morris R.E., Wuttke S.*, Toxicity of Metal-Organic Framework Nanoparticles: From Essential Analyses to Potential Applications, Chem. Soc. Rev., 2022, 51, 464-484.
Kuhn J., Lin Y., Krhac Levacic A., Al Danaf N., Peng L., Höhn M., Lamb D.C., Wagner E., Lächelt U.*, Delivery of Cas9/sgRNA Ribonucleoprotein Complexes via Hydroxystearyl Oligoamino Amides, Bioconj. Chem., 2020, 31(3), 729-742.
Kuhn J., Klein P.M., Al Danaf N., Nordin J.Z., Reinhard S., Loy D.M., Höhn M., El Andaloussi S., Lamb D.C., Wagner E., Aoki Y., Lehto T., Lächelt U.*, Supramolecular Assembly of Aminoethylene-Lipopeptide PMO Conjugates into RNA Splice-Switching Nanomicelles, Adv. Funct. Mater., 2019, 29, 1906432.
Steinborn B., Hirschle P., Höhn M., Bauer T., Barz M., Wuttke S., Wagner E., Lächelt U.*, Core-Shell Functionalized Zirconium-Pemetrexed Coordination Nanoparticles as Carriers with a High Drug Content, Adv. Therap., 2019, 2, 1900120.
Zimpel A., Danaf N., Steinborn B., Kuhn J., Höhn M., Bauer T., Hirschle P., Schrimpf W., Engelke H., Wagner E., Barz M., Lamb D.C., Lächelt U.*, Wuttke S.*, Coordinative Binding of Polymers to Metal–Organic Framework Nanoparticles for Control of Interactions at the Biointerface, ACS Nano, 2019, 13(4), 3884-3895.
Röder R., Preiß T., Hirschle P., Steinborn B., Zimpel A., Höhn M., Rädler J.O., Bein T., Wagner E., Wuttke S.*, Lächelt U.*, Multifunctional nanoparticles by coordinative self-assembly of His-tagged units with metal-organic frameworks, J. Am. Chem. Soc., 2017, 139(6), 2359–2368.
Hall A., Lächelt U., Bartek J., Wagner E.*, Moghimi S.M.*, Polyplex Evolution: Understanding Biology, Optimizing Performance, Mol. Ther., 2017, 25(7):1476-1490.
Liu X., Zhang P., He D., Rödl W., Preiß T., Rädler J.O., Wagner E., Lächelt U.*, pH-Reversible Cationic RNase A Conjugates for Enhanced Cellular Delivery and Tumor Cell Killing, Biomacromolecules, 2016, 17, 173-182.
Zimpel A., Preiß T., Röder R., Engelke H., Ingrisch M., Peller M., Rädler J.O., Wagner E., Bein T., Lächelt U.*, Wuttke S.*, Imparting Functionality to MOF Nanoparticles by External Surface Selective Covalent Attachment of Polymers, Chem. Mater., 2016, 28, 3318-3326.
Lächelt U.*, Wagner E.*, Nucleic Acid Therapeutics Using Polyplexes: A Journey of 50 Years (and Beyond), Chem. Rev. (Washington, DC, U. S.) 2015, 115, 11043-11078.
